Units
Molarity (M) is in moles solute per liter of solution: molsolute/Lsolution or mol/L
Cation Exchange Capacity (CEC) is often expressed in milliequivalents per 100g soil: meq/100g
Constants
Dissociation constant of water
Kw = [H+][OH–] = 1×10-14 at 25°C
Note: For pure water, [H+] = [OH–] = 1×10-7
Chemistry
In this section:
Base Saturation as %
Cation Charge from Soil Test Results
pH from [H+] & Vice Versa
Base saturation as %
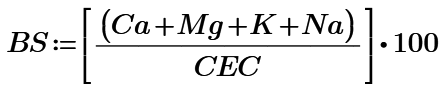
Where:
BS is the base saturation, as a percentage
Ca is the charge occupied by calcium ions in meq/100g soil
Mg is the charge occupied by magnesium ions in meq/100g soil
K is the charge occupied by potassium ions in meq/100g soil
Na is the charge occupied by sodium ions in meq/100g soil
CEC is the cation exchange capacity of the soil in meq/100g soil
cation Charge from Soil Test Results
Step 1: Determine gram equivalent weight for each cation of interest.

Where:
GEW is the gram equivalent weight
AW is the atomic weight of the cation
V is the charge, or valence, of the cation
Step 2: Convert the gram equivalent weight into meq/100g soil. For each cation, this is a constant that will not change. The gram equivalent weight is multiplied by ten because there are 10meq per gram of soil.

Where:
X is the conversion factor from ppm to charge in meq/100g soil
GEW is the gram equivalent weight
Step 3: Divide soil test levels (in ppm) by constant to find charge.

Where:
C is the charge contributed by the specific cation
R is the result as recorded in the soil test, in ppm
X is the conversion factor from ppm to charge
For a more detailed explaination of this conversion and the conversion factors of all the base cations, see this fact sheet from Ohio State University Extension.
pH from [H+] & vice versa
pH = -log[H+]
[H+] = 10-pH
Where:
[H+] is the molarity of H+ in solution
Physics
Darcy’s Law
q = K * ΔH
Where:
q is the discharge per unit flow (rate)
K is the hydraulic conductivity of the soil (units are distance/time)
ΔH
Stokes’s Law
Conversions
| Convert From: | To: | Equation: |
|---|---|---|
| meq/100g | mmol/kg | meq/100g x 10 = mmol/kg |